Innovation In a world where medical breakthroughs often hinge on novel discoveries, Paradigm Biopharmaceuticals is proving that innovation can also come from reimagining the old. With a drug that’s been used for over 60 years, Paradigm is poised to disrupt the osteoarthritis (OA) treatment landscape—and potentially become a multi-billion-dollar company in the process.
Repurposing a Proven Drug for a Modern Epidemic
Paradigm’s flagship compound, pentosan polysulfate sodium (iPPS), has long been used to treat inflammation, pain, and thrombosis in humans. It’s also a staple in veterinary medicine, where it’s successfully used to treat arthritis in dogs. Now, Paradigm is repurposing this trusted molecule to tackle one of the most debilitating and underserved conditions in human medicine: osteoarthritis.
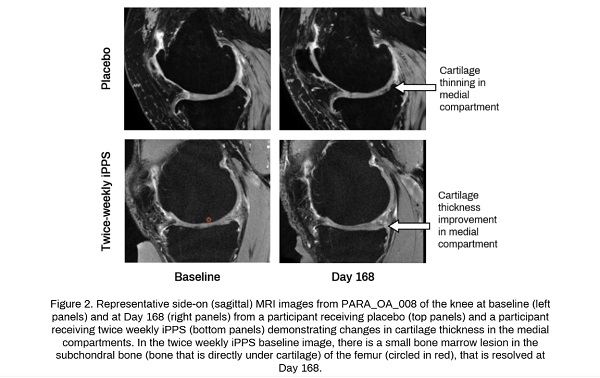
Clinical Trials That Could Change Everything
Paradigm’s PARAOA008 trial delivered results that are not just promising—they’re potentially transformative. Highlights included:
- Statistically significant improvements in pain and function at Day 56.
- Multiple signals of DMOAD efficacy (Disease-Modifying Osteoarthritis Drug) at Day 168 following a 6-week treatment course.
- MRI evidence of structural improvements in cartilage loss, bone marrow lesions, and marginal osteophytes.
- Favorable biomarker shifts in ARGS, C2C, COMP, and CTX II—key indicators of cartilage health. - Durable clinical responses across WOMAC pain, stiffness, and function scores. These results suggest that iPPS doesn’t just mask symptoms—it may actually modify the disease itself.
Why DMOAD Status Matters
Osteoarthritis affects millions globally, yet there are currently no approved DMOAD therapies. That’s a staggering gap in care, especially considering:
- 81% of OA patients are dissatisfied with current treatments.
- Physicians would likely adopt iPPS as a first-line therapy if DMOAD status is confirmed.
- DMOAD designation could significantly increase the price per treatment course, boosting Paradigm’s revenue potential.
Multi-Billion Dollar Potential—Even Without DMOAD Status
While DMOAD designation would be a game-changer, it’s important to recognize that Paradigm’s drug, iPPS, holds immense commercial value regardless of that outcome.
Here’s why:
- Proven Symptom Relief: Clinical trials have already demonstrated statistically significant improvements in pain, stiffness, and function. These benefits alone make iPPS a highly attractive option for the millions suffering from OA.
- Unmet Market Demand: With over 80% of OA patients dissatisfied with current treatments, there’s a massive opportunity for a safer, more effective alternative—even if it’s not disease-modifying.
- Veterinary Success: iPPS is already widely used in veterinary medicine to treat arthritis in dogs, reinforcing its safety and efficacy profile. This cross-species success adds credibility and market versatility.
- First-Line Therapy Potential: Physicians may still adopt iPPS as a first-line therapy based on its clinical performance and tolerability, especially for patients who cannot tolerate NSAIDs or opioids.
- Global Expansion: Paradigm’s Fast Track designation and international patent protections position the company to expand into global markets, multiplying its revenue potential.
- Pricing Power: Even without DMOAD status, the drug’s unique mechanism and durable symptom relief could justify premium pricing compared to existing therapies.
In short, Paradigm doesn’t need DMOAD status to succeed—it just needs to continue proving what it already has: that iPPS works, is safe, and fills a critical gap in OA treatment. With the right commercial strategy, partnerships, and regulatory momentum, Paradigm could still become a billion-dollar enterprise.
Fast Track and FDA Momentum
Paradigm has already secured Fast Track designation from the FDA, a critical step toward accelerated approval. While their first FDA trial didn’t yield optimal results, it wasn’t due to the drug’s efficacy—it was a dosing issue. The FDA initially required Paradigm to use lower doses than in its previous successful trial. This requirement set by the FDA proved suboptimal. However, past trials using 2mg/kg twice weekly for 6 weeks showed consistent improvements in cartilage thickness and clinical outcomes.
The current trial (PAR-002) includes revised dosing protocols that align with Paradigm’s historically proven regimen:
This refined approach is expected to unlock the full potential of iPPS.
Intellectual Property and Manufacturing Strength
Paradigm holds patents for Zilosul, their proprietary formulation of iPPS, and has secured a 25-year agreement with its manufacturer. This ensures long-term supply chain stability and protects their market position as they scale.
The Bottom Line
Paradigm Biopharmaceuticals isn’t just another biotech hopeful—it’s a company with a proven molecule, compelling clinical data, and a clear path to regulatory approval. With no current DMOAD therapies on the market, Paradigm is uniquely positioned to fill a massive unmet need.
And even if DMOAD status isn’t achieved, the company’s drug still offers a powerful, differentiated solution for OA patients worldwide. For investors seeking exposure to a company with real potential to revolutionize OA treatment and capture a multi-billion-dollar market, ASX: PAR deserves serious attention.
This is not financial advice. Please do your own research before making investment decisions.
Citations
Clinical Trials and Drug Data - PARAOA008 trial summary (Paradigm Biopharma investor presentation): https://paradigmbiopharma.com/investor-centre/presentations/
ClinicalTrials.gov entry for PAR-002 (current FDA trial): https://clinicaltrials.gov/study/NCT05727670
Regulatory and Market Data - FDA Fast Track designation overview: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track
Global osteoarthritis market forecasts: Example source with projections: https://www.grandviewresearch.com/industry-analysis/osteoarthritis-therapeutics-market
Drug Mechanism and Veterinary Use - iPPS in veterinary medicine (e.g. Cartrophen Vet): https://www.cartrophen.com.au/vet
Biomarkers and DMOAD Overview - Disease-Modifying Osteoarthritis Drug (DMOAD) basics (Osteoarthritis Research Society International): https://oarsi.org
Relevant biomarkers in OA research (COMP, ARGS, CTX-II): https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6348512/
Intellectual Property and Manufacturing - Paradigm’s Zilosul patents (via IP Australia or company announcements): https://paradigmbiopharma.com/news/
Media Contact
Company Name: Fact Checked Media
Contact Person: Sam Yutuc
Email: Send Email
Phone: +61 461 366 337
Country: Australia
Website: https://www.factcheckedmedia.com/
