Articles from Qbtech

As demand for virtual ADHD care increases, findings from a new study conducted with ADHD 360, the UK’s largest evidence-based digital service specializing in ADHD diagnosis and treatment, reveal how objective ADHD diagnostic and monitoring technology improves patient outcomes and clinical certainty.
By Qbtech · Via Business Wire · May 10, 2025

Legion Health, a telepsychiatry provider dedicated to transforming patient care through advanced technology, announces its clinic-wide adoption of QbCheck, an FDA-cleared remote objective ADHD testing technology from Qbtech. As a completely virtual clinic, Legion Health recognized the need for standardization and efficiency in ADHD care and the benefits QbCheck could provide.
By Qbtech · Via Business Wire · April 8, 2025

The ADHD Expert Consortium, a diverse group of professionals advocating for improved ADHD care, held its fourth annual meeting in Houston, Texas. This year’s meeting focused on discussions around standardization and consistency in ADHD assessments, technologies to improve ADHD care and management, and the role patients play in their care.
By Qbtech · Via Business Wire · February 13, 2025

Arizona ADHD Center, a medical and behavioral health clinic specializing in ADHD diagnosis, treatment, and ongoing maintenance with locations in the Phoenix community, announces the opening of its Scottsdale clinic. The new center treats adult and pediatric ADHD patients and brings more than 10 years of ADHD experience to the Scottsdale area.
By Qbtech · Via Business Wire · January 28, 2025

Renewing Hope Family Counseling Center, Inc., a group of California-based mental health outpatient clinics for individuals, families and couples, announces its adoption of objective ADHD testing technology from Qbtech. Renewing Hope, Inc. has implemented Qbtech, a provider of FDA-cleared solutions for ADHD diagnostic and treatment monitoring, across multiple mental health clinics to address high-need, low-resource populations in Southern California. This partnership helps address the area’s critical shortage of care options for ADHD management among children and adults with ADHD symptoms.
By Qbtech · Via Business Wire · November 12, 2024

Qbtech, the global leader in objective ADHD testing, today announced its one millionth patient milestone, amassing one of the most extensive data sets of patients tested for ADHD. Adopted by more than 12,000 healthcare providers worldwide, including 44 U.S. states, the company’s industry-leading tests provide evidence-based data for unbiased decision-making and enhanced patient care. Qbtech’s objective ADHD tools, QbTest and QbCheck, are FDA-cleared solutions for ADHD diagnostic and treatment support in children, adolescents and adults.
By Qbtech · Via Business Wire · October 29, 2024

Qbtech, the global leader in objective ADHD testing, today announced the launch of its new grant program, QbAccess. Created to make ADHD testing and care more accessible to underserved populations, the program provides non-profit healthcare organizations with access to Qbtech’s FDA-cleared objective ADHD testing devices at a reduced rate.
By Qbtech · Via Business Wire · October 15, 2024

Qbtech, the global leader in objective ADHD testing, today announces the publication of a new manuscript, “Utilizing remote objective ADHD testing to monitor symptom improvement following medication treatment,” in the International Journal of Psychiatry Research 2024. This manuscript focuses on the use of QbCheck, its FDA-cleared remote objective testing technology, for monitoring ADHD treatment effectiveness and symptom improvement. The research was completed by Ragini Sanyal, Robert Nolen, Urban Gustafsson, and Dr. Mikkel Hansen. The full manuscript is available here.
By Qbtech · Via Business Wire · August 13, 2024

The National Institute for Clinical Excellence (NICE), a UK government-funded, non-departmental public body that aims to establish guidelines for clinical best practice, issued its recommendation for the National Health Service (NHS) to use Qbtech’s industry-leading objective tests to improve diagnostic wait times for children and youth, age six to 17. The approval for using QbTest alongside traditional diagnostic methods enables more children to get diagnosed within six months of their initial assessment, addressing the long waiting periods many families are experiencing.
By Qbtech · Via Business Wire · July 30, 2024

In response to the recent arrests of Done founder Ruthia He and clinical leader David Brody for the company’s alleged stimulant prescription practices, Qbtech, the global leader in objective ADHD testing, announced today it is waiving testing fees for former Done patients. The free objective tests are designed to serve as a safeguard for patients seeking a re-assessment to validate an ADHD diagnosis completed by a Done virtual provider.
By Qbtech · Via Business Wire · July 15, 2024

The ADHD Expert Consortium, a diverse group of professionals advocating for improved ADHD care, held its third annual meeting in Houston, Texas.
By Qbtech · Via Business Wire · May 2, 2024

Qbtech, a leading ADHD health-tech company, has collaborated with BBC journalist Simon Mundie to produce Rethinking ADHD, a podcast to educate the public about the condition through expert commentary and real-life experiences. The series addresses the current state of ADHD, tackles contemporary myths around the condition, and focuses on different symptoms and how people can be affected.
By Qbtech · Via Business Wire · November 1, 2023

Today, industry leaders convened at a congressional briefing, focused on youth mental health titled, “Improving Outcomes in Youth Mental Health: What's Working and What Can Be Done."
By Qbtech · Via Business Wire · October 25, 2023

ADHD, a highly treatable neurological disorder, affects millions of people in the United States. National prevalence rates rose consistently between 1997-2016, increasing from 6.1% to 10.2% according to a population survey. Despite the common perception that ADHD is overdiagnosed, the opposite is, in fact, true. Increasing rates of ADHD are not due to over-care but rather increased awareness of the condition.
By Qbtech · Via Business Wire · June 12, 2023

Qbtech, the global leader in objective ADHD testing, today announces the opening of its new U.S. headquarters in Houston, Texas. Founded in Stockholm, Sweden, and with operations across the UK and Europe, the company has rapidly increased its market share in the U.S., with recent investment from specialist growth equity investor Verdane fueling further growth. The 6,000 sq. ft. Houston office will serve as the base for all U.S. operations and house several departments including medical research, quality, product, engineering, marketing, technical support and human resources, helping expand the company’s presence.
By Qbtech · Via Business Wire · May 3, 2023

Last month, the Department of Justice through the Drug Enforcement Administration (DEA), in consultation with the Departments of Health and Human Services (HHS), released a Notice of Proposed Rulemaking (NPRM) that, if finalized, would reinstate the in-person visitation requirements for physicians to prescribe patients classified medications, including Adderall. Qbtech strongly supports patients' access to high-quality health care and believes the current DEA rule, although an improvement to care, is far too restrictive. The pandemic demonstrated that flexibility in how and where patients receive care promotes improved outcomes. Qbtech stands with its providers and will continue to work with them to ensure patients receive the proper diagnosis and treatment.
By Qbtech · Via Business Wire · March 27, 2023

The National Institute for Clinical Excellence (NICE), a UK government-funded, non-departmental public body that aims to establish guidelines for clinical best practice, recognizes QbTest in its MedTech Innovation Briefing, MIB318. The Medical Innovation Briefing highlights the benefits of Qbtech’s objective testing technology when used as part of a comprehensive ADHD assessment.
By Qbtech · Via Business Wire · March 9, 2023

The ADHD Expert Consortium, a diverse group of ADHD professionals, held its second annual meeting in Orlando, Fla., to expand on its calls to action for improved ADHD diagnosis and care in the United States. In addition to its 12 founding members, the ADHD Expert Consortium added six new members this year, offering additional perspectives on improving the care pathway for ADHD patients.
By Qbtech · Via Business Wire · January 26, 2023

The Focus ADHD program, created by objective testing provider Qbtech and the AHSN Network, received the Best Innovative Mental Health Invention at the 2022 National Mental Health and Wellbeing Awards. Earlier this year, Qbtech and the AHSN network were winners of the prestigious ‘Best Mental Health Partnership with the NHS' at the HSJ awards.
By Qbtech · Via Business Wire · November 17, 2022

Today, Qbtech, the software provider of objective ADHD tests QbTest and QbCheck, announces an investment by specialist growth equity investor, Verdane. Qbtech is the market leader in objective measurement of ADHD symptoms, transforming ADHD care for individuals and society at large. Working with specialists in health and education in the US and Europe, Qbtech reduces delay to diagnosis and enables treatment optimization for children and adults with ADHD. Verdane’s investment will support Qbtech to further accelerate growth through new technologies, new customer segments and through significant expansion in existing markets.
By Qbtech · Via Business Wire · August 29, 2022

Qbtech and The AHSN Network have been shortlisted for the 2022 Health Service Journal award for Best Partnership for QbTest, an objective assessment used in ADHD diagnosis.
By Qbtech · Via Business Wire · August 10, 2022

The ADHD Expert Consortium, a group of ADHD experts comprised of psychiatrists, psychologists, neurologists, pediatricians, nurse practitioners, neuropsychologists and patient advocates, has issued a consensus statement detailing needed improvements for the future of ADHD care in the United States. The ADHD Expert Consortium met in Dallas, Texas, earlier this year to discuss key issues facing providers, patients and key stakeholders.
By Qbtech · Via Business Wire · July 6, 2022

Today, Qbtech announces a partnership in The Netherlands with one of the leading ADHD care providers, ADHDcentraal. The purpose of the collaboration is to create new methods and tools that allow patients to access high-quality care readily, something that is not possible today.
By Qbtech · Via Business Wire · June 22, 2022

Qbtech and The AHSN Network received the Health Service Journal award for Best Mental Health Partnership for QbTest, an objective assessment used in ADHD diagnosis. The award comes after The AHSN Network’s ongoing support of the digital innovation that has prompted nearly 57,000 people between the ages of 6 and 18 to receive objective ADHD testing from the National Health Service in the UK.
By Qbtech · Via Business Wire · March 28, 2022

Qbtech, the technology-first leader in ADHD assessment and testing, today announced the launch of its U.S. Telehealth Map. Available at www.qbtech.com/telehealth-adhd, the interactive resource will serve as a reference tool for psychiatrists, pediatricians, nurse practitioners and other ADHD clinicians seeking to expand care opportunities across their state.
By Qbtech · Via Business Wire · November 16, 2021
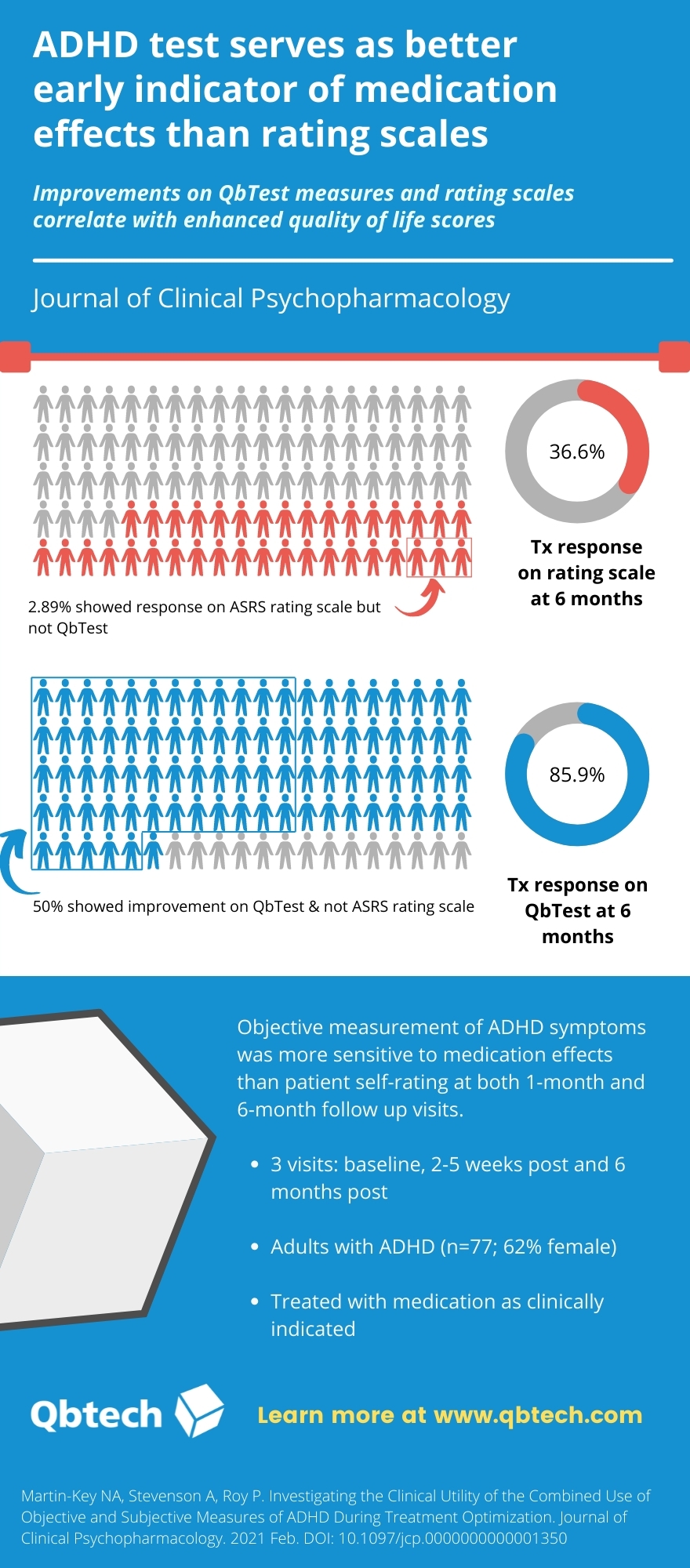
A study published in the Journal of Clinical Psychopharmacology found that an FDA cleared, computer-based, objective measurement of attention-deficit/hyperactivity disorder (ADHD) symptoms was more sensitive to medication effects than patient self-rating, at both one-month and six-month follow-up visits. The Quantified Behavioral Test (QbTest), serves as a better early indicator of treatment effects for ADHD, confirming that healthcare professionals can confidently use objective data alongside patient feedback regarding treatment effectiveness.
By Qbtech · Via Business Wire · June 8, 2021
